Abstract
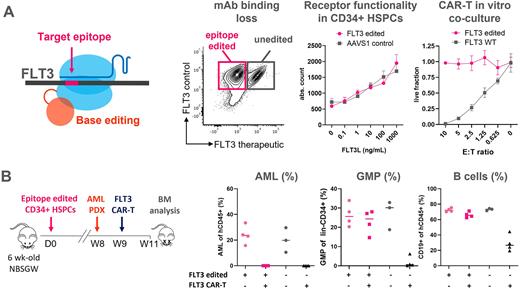
Despite treatment advances and the use of allogeneic hematopoietic stem/progenitor (HSPC) cell transplantation (HSCT), acute myeloid leukemia (AML) is still associated with an unfavorable outcome for >50% of patients. Whereas novel immunotherapies, such as CAR-T cells, bispecific and toxin conjugated antibodies (Ab), demonstrated clinical efficacy when targeting dispensable lineage antigens (Ag), such as CD19 in B-ALL and lymphomas, the same approach cannot be exploited for AML, due to lack of actionable leukemia-restricted Ags. Suitable targets are shared with healthy progenitor or mature myeloid cells, leading to on-target off-tumor toxicity and impairment of hematopoietic reconstitution. Despite this, anti-AML immunotherapies are currently under development, but their use is restricted to a limited time window, which is likely insufficient for disease eradication. To address this issue, we reasoned that precise gene modification of the targeted epitopes in donor HSPC used in HSCT would result in loss of recognition, without affecting normal protein expression, regulation, and function. Differently from the abrogation of dispensable lineage markers (ie. CD33), epitope editing allows targeting genes essential for leukemia survival regardless of shared expression or functional role in normal HSPC, thus minimizing the risk of tumor immune escape by Ag loss or downregulation. As targets, we selected the cytokine receptors FLT3, KIT and CD123 (IL3RA), which are found in >85% of AML cases and whose mutation or overexpression is associated with poor prognosis. By transposon-based library screenings, we identified amino acid substitutions in the FLT3, KIT and CD123 extracellular-domains (ECD) that preserve physiologic surface expression, ligand-binding and intracellular kinase activation but avoid detection by a therapeutic monoclonal Ab (panel A). Cells expressing these variants were resistant to CAR-T killing and did not induce CAR-T activation and proliferation during in vitro co-culture (panel A). We identified a set of gRNAs that enable the introduction of these mutations by an advanced generation adenine base editor (ABE) without the need for dsDNA breaks. Electroporation of ABE8e mRNA and gRNAs into human CD34+ HSPCs achieved up to 90%, 85% and 75% editing efficiency on FLT3, KIT and CD123 genes, respectively, either as single or multiplex editing. After xenotransplant into NBSGW mice, FLT3, KIT or CD123 epitope editedHSPC sustained long-term multilineage hematopoiesis indicating editing of the HSC compartment. Upon treatment with anti FLT3 CAR-T, we observed sparing of human CD34+38- HSPCs, granulo-mono progenitors (GMP), B cells and B-progenitors in the bone marrow of mice engrafted with FLT3-edited HSPCs compared to AAVS1 controls (panel B). Similarly, treatment with CD123 CAR-T cells showed protection of epitope-edited myeloid lineages, including granulocytes, DC and HSPCs. Additionally, we confirmed the selective resistance of epitope-edited HSPCs and their progeny in mice co-engrafted with human patient-derived AML xenografts (PDX), which were eradicated by FLT3 or CD123 CAR-T. In conclusion, transplantation of epitope engineered HSPCs endowed with selective resistance to CAR T cells or Abs is a novel approach to enable more effective and safer immunotherapies for high-risk AML patients. Furthermore, this approach can be exploited as non-genotoxic conditioning to allow engraftment and in vivo selection of genome engineered cells, without the need for chemo- or radiotherapy myeloablation.
Disclosures
Rambaldi:ABBVIE: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Astellas: Honoraria; Omeros: Honoraria; Celgene-BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria. Ritz:Equillium: Research Funding; Gadeta: Research Funding; Kite/Gilead: Research Funding; Oncternal: Research Funding; Novartis: Research Funding; Akron Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; AvroBio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Clade Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Draper: Consultancy, Membership on an entity's Board of Directors or advisory committees; Garuda Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; LifeVault Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Smart Immune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Talaris Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Armstrong:Cyteir Therapeutics: Consultancy, Other: Shareholder; C4 Therapeutics: Consultancy, Other: SHareholder; Accent Therapeutics: Consultancy, Other: Shareholder; Twenty eight-seven Therapeutics: Consultancy, Other: Shareholder; Mana Therapeutics: Consultancy, Other: Shareholder; Janssen: Research Funding; Novartis: Research Funding; Syndax: Research Funding; -: Patents & Royalties: MENIN inhibition WO/2017/132398A1; Imago Biosciences: Consultancy, Other: Shareholder; Neomorph Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal